Scientists Reveal the Mechanism of Phosphate Distribution by SULTR family Transporter SPDT in Rice
On October 11 2025, Peng Zhang’s group from the Center for Excellence in Molecular Plant Sciences of the Chinese Academy of Sciences and Fang Yu’s group from the College of Life Sciences at Shanghai Normal University published an article entitled “Molecular mechanism underlying phosphate distribution by SULTR family transporter SPDT in Oryza sativa” in the international academic journal Science Advances. The study determined the three-dimensional structure of SPDT, a key phosphate distribution transporter in rice, revealing the molecular mechanism of its specific phosphate transport.
Phosphorus is an essential macronutrient for plant growth and development. However, the availability of phosphorus in the form of inorganic phosphate in soil is typically limited, which constitutes a primary constraint on crop productivity and represents a significant challenge in global agricultural systems. Plants rely on multiple inorganic phosphate transporters to accomplish phosphorus uptake, translocation, and distribution: PHT1 family proteins mediate phosphorus absorption from the soil; PHO1 facilitates the phosphorus translocation from root to shoot; and SPDT regulates phosphate partitioning, thereby influencing subsequent allocation to developing grains; VPE and PHT2/3/4/5 proteins are involved in the regulation of phosphorus homeostasis at the subcellular level (Fig. 1).
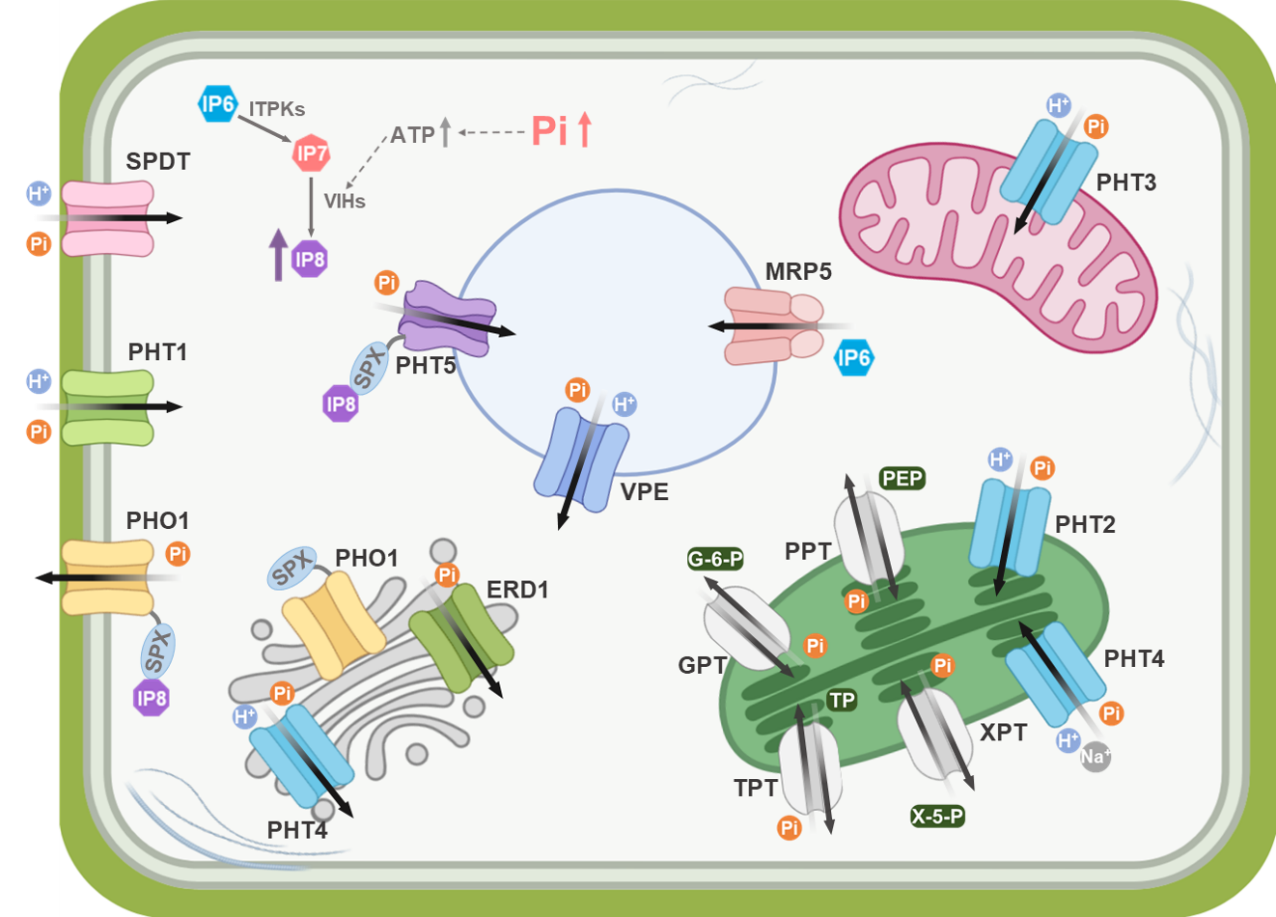
Figure 1. Plant Pi transport system
Although these transporters play crucial roles in plant phosphorus homeostasis, their structures and molecular mechanisms remain largely unknown. In previous work, the team determined the three-dimensional structure of the Arabidopsis phosphate exporter PHO1;H1, revealing its mechanism of phosphate transport and its regulation by inositol polyphosphate (InsP) signaling molecule (Fang et al, Nat Plants, 2025, doi: 10.1038/s41477-024-01895-6).
The present study focuses on OsSPDT (SULTR3;4), a key phosphate distribution transporter in rice. Although phylogenetically classified within the sulfate transporter (SULTR) family, SPDT exhibits distinct specificity for phosphate transport. However, the evolutionary and mechanistic basis for its functional shift from sulfate to phosphate transport remained poorly understood.
This study first verified the specific phosphate transport function of SPDT through yeast heterologous complementation assays, confirming that it does not possess sulfate transport capability (Fig. 2A). Subsequently, the research team employed single-particle cryo-electron microscopy to determine high-resolution three-dimensional structures of OsSPDT in both the phosphate-bound and apo states. The structures revealed that OsSPDT exists as a homodimer, with each monomer consisting of an N-terminal intracellular domain (NTD), a transmembrane domain (TMD), and a C-terminal STAS domain (Fig. 2B). The TMD can be further divided into Core and Gate subdomains, with the phosphate-binding site located at their interface. Structure-based analysis and functional assays revealed that replacing the TMD of SULTR with that of OsSPDT conferred sulfate transport activity to OsSPDT. Further analysis identified Ser170 on TM3 as a signature residue within the SPDT family at the binding site. A single-site mutation at this position resulted in the loss of phosphate transport function but did not confer the ability to transport sulfate, suggesting that the specificity for phosphate recognition likely depends on the co-evolution of the entire TMD.
The study also found that the NTD and STAS domains are crucial for maintaining dimer stability and transport activity. Truncation experiments showed that the 49–55 amino acid segment within the NTD is a key region for maintaining dimerization and function (Fig. 2C). The STAS domain stabilizes the TMD of the adjacent monomer through electrostatic interactions and may participate in regulating conformational changes during transport.
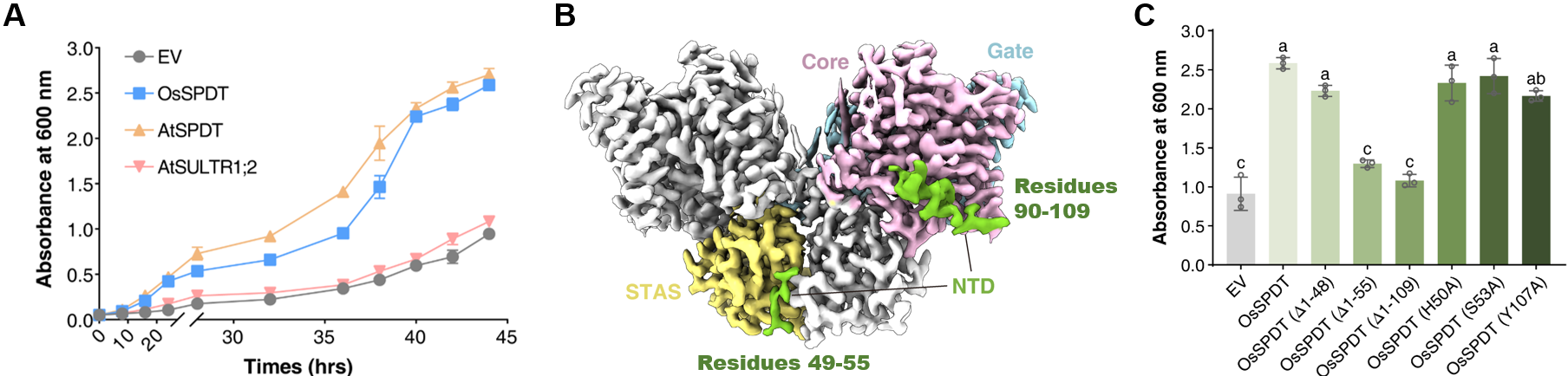
Fig. 2 Structure and functional analysis of OsSPDT
Thus, the three-dimensional structures, substrate recognition/transport, and regulatory mechanisms of two distinct families of inorganic phosphate transporters in plants—PHO1;H1 (SPX-EXS family) and SPDT (SULTR family)—have been thoroughly elucidated. Given the critical roles these proteins play in long-distance phosphorus transport (PHO1) and tissue distribution (SPDT), this study not only reveals the molecular mechanisms underlying phosphorus homeostasis regulation in plants but also lays the groundwork for developing crop varieties with low phytic acid and high phosphorus use efficiency (Figure 3).
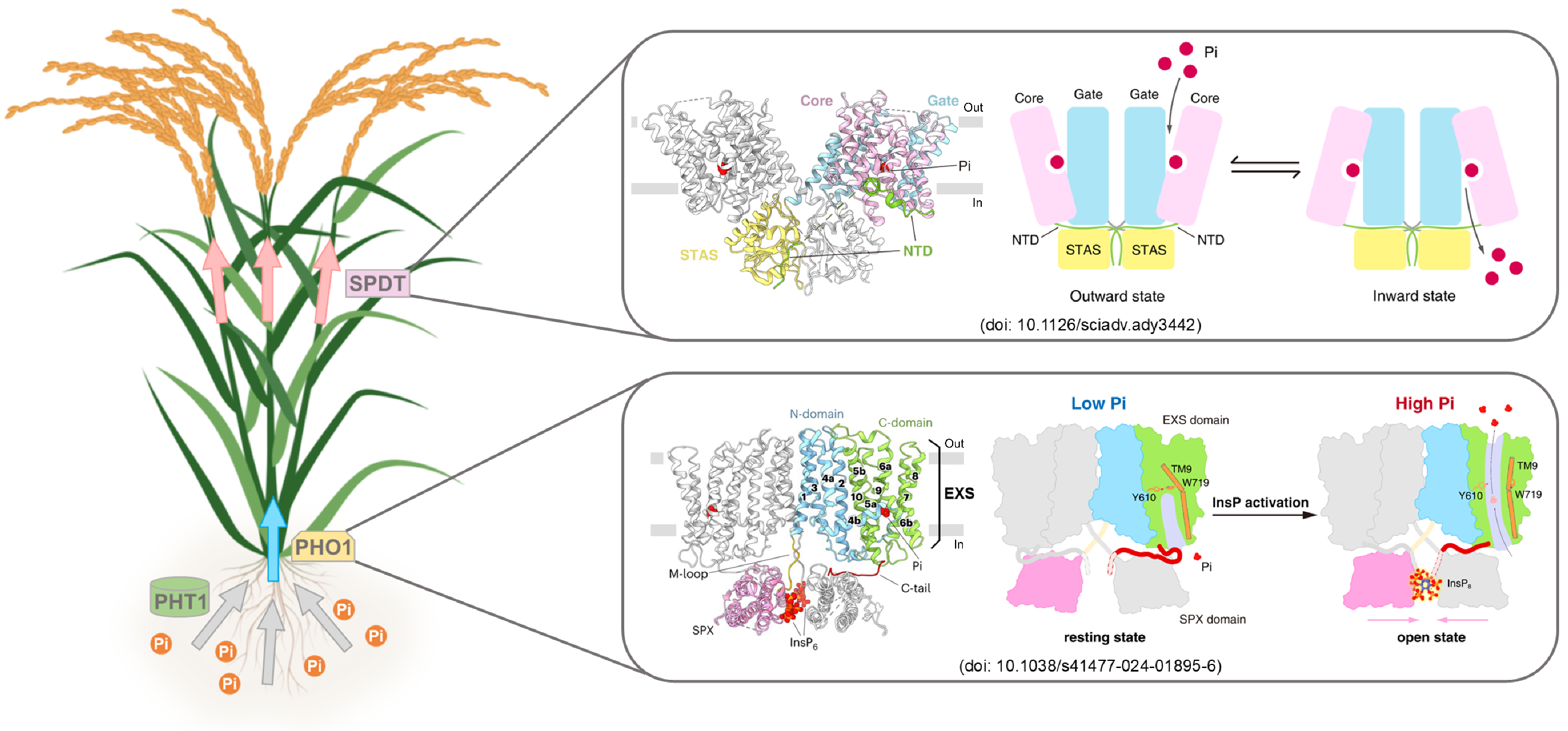
Fig. 3 The working model for SPDT and PHO1
Dr. Sunzhen Fang, a postdoctoral fellow at the Center for Excellence in Molecular Plant Sciences of the Chinese Academy of Sciences, is the first author of this paper. Yang Zhao (a graduated master's student) from the College of Life Sciences at Shanghai Normal University and Dr. Xue Zhang, an assistant researcher in Zhang Peng’s group, also participated in the research. Prof. Peng Zhang and Prof. Fang Yu are the corresponding authors. This work was supported by grants from the National Natural Science Foundation of China, the Chinese Academy of Sciences and the Shanghai Municipal Project.
Article Link: https://www.science.org/doi/10.1126/sciadv.ady3442
Contact: pengzhang01@cemps.ac.cn