The Origin and Evolution of ABA Receptor PYLs: Discoveries by CAS Scientists
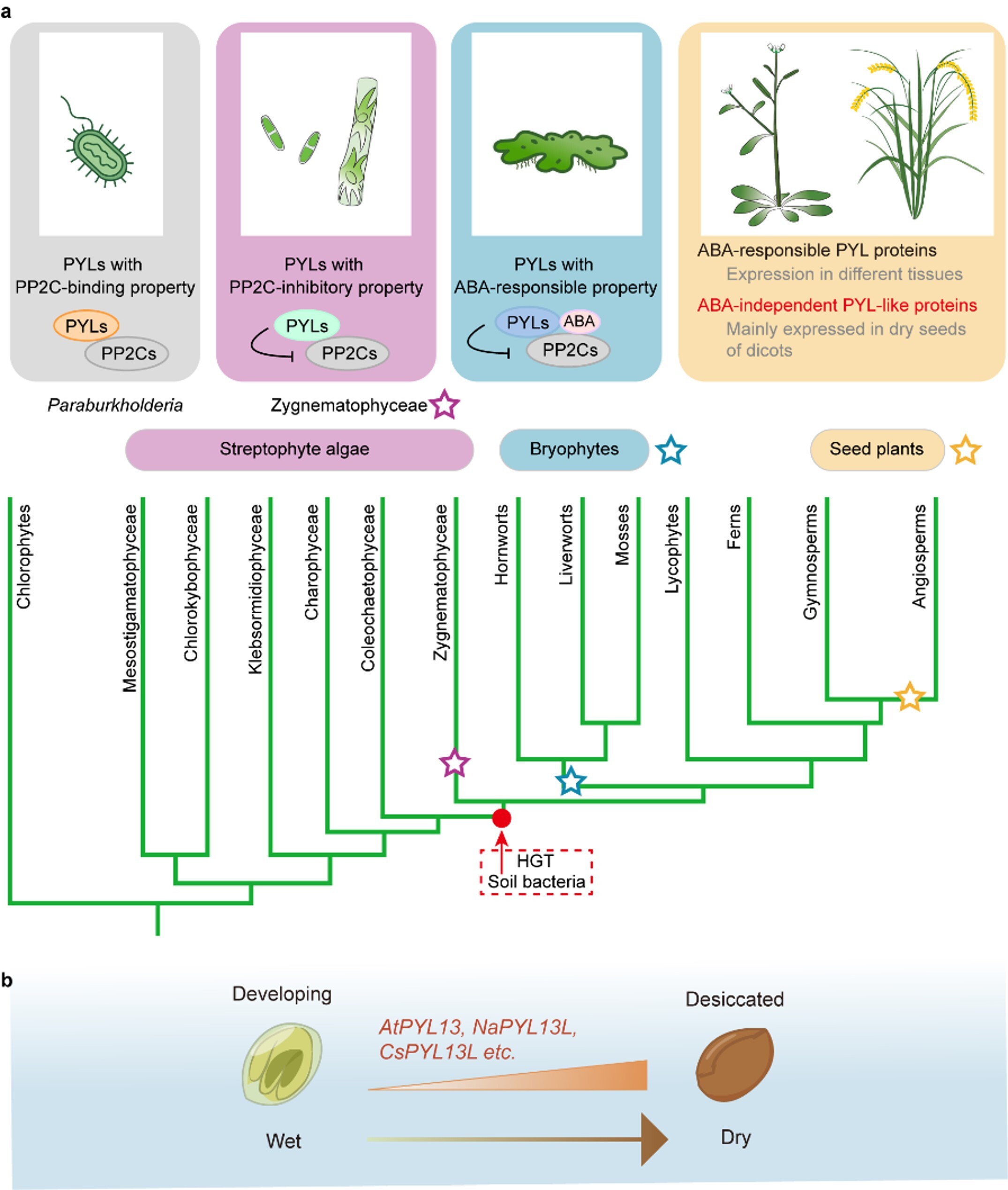
Terrestrialization represents a pivotal event in green plant evolution, requiring adaptive mechanisms to overcome water stress. Plants primarily rely on the ABA signaling pathway to implement adaptive strategies such as “drying without dying (desiccation tolerance)” or “avoiding drying (drought avoidance)”. Upon binding ABA, PYL family receptors inhibit the activity of clade A PP2C protein phosphatases, thereby activating SnRK2 kinases and their downstream response pathways. The ABA-binding properties of PYLs vary significantly; likewise, their PP2C inhibition mechanisms show distinct ABA-dependence patterns: (i) strictly ABA-dependent (no basal inhibition activity without ABA); (ii) ABA-enhanced (basal inhibition activity potentiated by ABA); and (iii) ABA-independent (not modulated by ABA, including constitutively active and constitutively inactive types). However, the origin, evolution, distribution, and specific functions of ABA-independent PYL-like proteins in land plants remain unclear.
Photosynthetic eukaryotic life originated in the ocean approximately 1.5 billion years ago. Subsequently, green algae gradually became established in freshwater ecosystems between about 1 billion to 500 million years ago; around 400 million years ago, the terrestrialization of freshwater green algae occurred. Previous genomic studies proposed the "horizontal gene transfer of PYLs" hypothesis but lacked experimental evidence.
Scientists from the CAS Center for Excellence in Molecular Plant Sciences (CEMPS) systematically identified ABA-independent PYL-like proteins from bacteria, green algae, and angiosperms. They found that during evolution, PYLs sequentially acquired three critical properties for becoming an ABA receptor: the PP2C-binding ability, the PP2C-inhibiting ability, and finally ABA-binding ability, thus fully revealing the key evolutionary path from ABA-independent PYL-like proteins to ABA receptors.
Specifically, the PrPYL protein from the nitrogen-fixing bacterium Paraburkholderia rhynchosiae can bind clade A PP2Cs but neither inhibits PP2C activity nor binds ABA, representing a class of “prototype PYLs”. Within some green algae of the order Zygnematales, there exist “prototype PYLs” within the plant kingdom that have acquired the ability to bind and inhibit PP2Cs but still cannot bind ABA. These ABA-independent PYL-like genes endowed green algae with ABA-independent stress response capabilities, which were critical for their subsequent terrestrialization. These findings provide experimental evidence for the “horizontal gene transfer of PYLs” hypothesis.
In the common ancestor of land plants, PYLs evolved the ability to bind and respond to ABA. However, ABA-independent PYL-like proteins did not completely disappear in land plants but underwent evolution from “receptors” to “pseudo receptors” in seed plants. Scientists from CEMPS identified several such proteins in angiosperms, including Arabidopsis AtPYL13, pepper CaPYL13L2, rice OsPYL12, barley HvPYL13L, etc. The ABA-independence of these proteins stems from variations in two key amino acid residues within their ABA-binding pocket. In dicot plants, these genes are predominantly highly expressed in seeds, a pattern regulated by the transcription factor ABI3. The study speculates that the ability of PYL-like proteins to constitutively activate stress signaling in seeds might contribute to maintaining seed vigor.
In summary, plant PYL genes originated from horizontal gene transfer from bacteria. In the common ancestor of land plants, this gene evolved from a " pseudo receptor" into an "ABA receptor" capable of binding and responding to ABA, thereby hijacking the conserved SnRK2-mediated osmotic stress signaling pathway. This transformation turned ABA from a secondary metabolite into a key plant stress hormone. Furthermore, in some seed plants, several PYLs underwent functional evolution from “receptors” back to “pseudo receptors”, which may aid in the long-term maintenance of seed vigor.
This study was published on Nature Communications on December 16, 2025. Prof. Yang Zhao from CEMPS is the corresponding author. Dr. Tianjiao Lu and Qingzhong Li of CEMPS are the co-first authors. This study was supported by the Biological Breeding-National Science and Technology Major Project (2023ZD040710202), the Project of Stable Support for Youth Teams in Basic Research of the Chinese Academy of Sciences (YSBR-119), Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB0630201).
Article Linkling: https://www.nature.com/articles/s41467-025
Contact: zhaoyang@cemps.ac.cn